作者: 刘珏 程和发

图文摘要 | Graphical abstract
导读 | Introduction
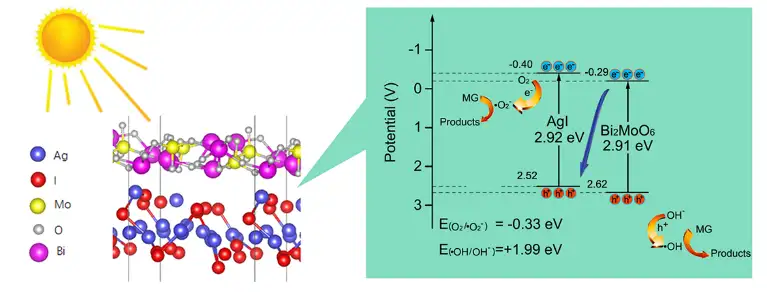
工农业生产造成的水污染已成为全世界水环境面临的主要挑战之一。光催化材料可以有效利用太阳能降解有机污染物,在水处理方面具有重要的应用前景。然而,单组分光催化剂光生载流子复合速率快、电荷转移效率低和自由基生成效率低,严重限制了其在水处理中的应用。为了解决该问题,本研究采用简单的原位生长法制备了由Bi2MoO6和AgI组成的Z型光催化剂,通过提高光吸收效率和降低光生载流子复合速率提高光催化性能。
Pollution resulting from the emissions of a wide range of industrial and agricultural activities has become one of the major environmental challenges in the world, particularly in the developing countries. Photocatalysis holds great promise in pollution control as it can degrade organic pollutants by utilizing solar energy. However, for single-component photocatalysts, the rapid recombination of photogenerated electron-hole pairs, poor charge transfer efficiency, and inefficient radical generation severely limit their applications in wastewater treatment. Based on this, a Z-type photocatalyst composed of Bi2MoO6 and AgI was prepared by a simple in-situ growth method, which possesses the merits of strong visible light absorption and low recombination efficiency of photogenerated electron-hole pairs induced by the formation of heterojunction.
一、光催化剂颗粒
纯Bi2MoO6呈花状双层结构,直径约为1μm(图1a)。由于不规则AgI薄片在Bi2MoO6上的原位生长,Bi2MoO6/AgI复合光催化剂表面显示出较为平滑的覆盖物,且Bi2MoO6两层之间的空隙逐渐被填充(图1b,1c,1d和1e)。BA11(Bi2MoO6与AgI的摩尔比为1:1)由于AgI的负载量较低,保持了纯Bi2MoO6的花状双层结构。EDS结果表明,Bi、Mo、O、Ag和I均匀分布在BA11上(图1i,1j,1k,1l,1m,1n)。
Figure 1 shows the surface morphology and microstructure of the as-prepared photocatalysts. Among them, BA21, BA11, BA12, and BA14 represents the nanocomposite with Bi2MoO6 to AgI molar ratio of 1:1, 1:2, 1:3, 1:4, respectively. Bi2MoO6 particles showed a flower-like bilayer morphology with a diameter of about 1 μm (Fig. 1a). Figure 1b–e depicts the morphology of Bi2MoO6/AgI nanocomposites. It can be seen that some AgI nanoparticles were uniformly dispersed on the surface of Bi2MoO6, while the rest penetrated into its flower-like hollow structure. In contrast, pure AgI occurred as irregular flakes (Fig. 1f). The TEM image of BA11 (Fig. 1g) indicates that it carried the flower-like spherical structure of Bi2MoO6. The results of elemental mapping showed that Bi, Mo, O, Ag, and I were uniformly distributed on the prepared photocatalyst (Fig. 1i–n).

图1 光催化剂的SEM和TEM图像
(a)Bi2MoO6,(b)BA21,(c)BA11,(d)BA12,(e)BA14和(f)AgI的 SEM图像;(g)BA11的TEM图像,(h)BA11的高分辨TEM图像和(i-n)BA11 的EDS谱图
Fig. 1 SEM and TEM images of photocatalysts.
SEM micrographs of (a) Bi2MoO6, (b) BA21, (c) BA11, (d) BA12, (e) BA14, and (f) AgI; (g) TEM micrograph and (h) high resolution TEM micrograph of BA11; and (i-n) elemental mapping of BA11
二、光催化剂的光学及光电化学性质
由紫外-可见漫反射可以看到,与单独组分相比,Bi2MoO6/AgI复合光催化剂吸收边明显红移,表明其对可见光的吸收增强(图2a),纯Bi2MoO6和AgI的能带分别为2.91和2.92 eV(图2b)。电化学测试表明,BA11的稳定光电流密度达到3.14 μA/cm2,远高于Bi2MoO6(0.45 μA/cm2)和AgI(1.75 μA/cm2), 这一结果可能是由BA11的电子与空穴复合速率较低,分离效率较高所致(图3a)。电化学阻抗谱表明BA11的电荷转移电阻较低,这是由Bi2MoO6和AgI界面处电荷的快速扩散所致(图3b)。莫特-肖特基曲线则进一步确定了Bi2MoO6和AgI的导带位置(图3c)。
Fig. 2a shows the UV-Vis diffuse reflectance spectra of the photocatalysts. Compared with the individual components, Bi2MoO6/AgI nanocomposites, especially when the one with Bi2MoO6 to AgI molar ratio of 1:1 (BA11), exhibited an obvious red shift in the absorption edge, which is indicative of enhanced absorption of visible light. The bang gaps of Bi2MoO6 and AgI were calculated to be 2.91 and 2.92 eV, respectively (Fig. 2b). Electrochemical characterization was applied to study the photoelectrochemical properties of the photocatalysts. The stable photocurrent density of BA11 reached 3.14 μA/cm2, which is much higher than that of Bi2MoO6 (0.45 μA/cm2) and AgI (1.75 μA/cm2) (Fig. 3a). Electrochemical impedance spectra indicated a decrease in charge transfer resistance in BA11, and the Mott-Schottky curve further confirmed the band structure of BA11 (Fig. 3b and c).

图2 Bi2MoO6、AgI、Bi2MoO6/AgI复合光催化剂的光学性能
(a)紫外-可见漫反射光谱 (DRS)和(b)带隙
Fig. 2 Optical characterization ofBi2MoO6, AgI, and Bi2MoO6/AgI nanocomposites.
(a) UV–Vis diffuse reflectance spectra (DRS); and (b) calculation of band gaps
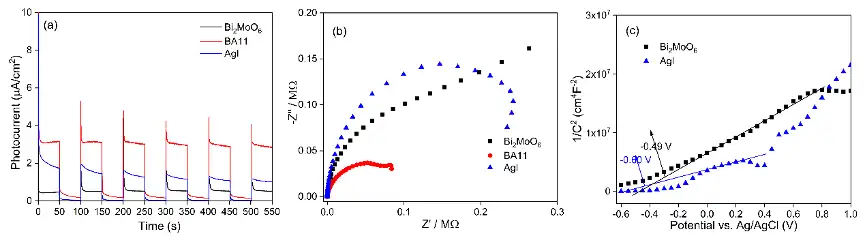
图3 Bi2MoO6、AgI和BA11 的光电化学表征
(a)瞬态光电流响应 (TPR);(b)电化学阻抗谱 (EIS)和(c)莫特-肖特基曲线
Fig. 3 Photoelectrochemical characterization of Bi2MoO6, AgI, and BA11.
(a) transient photocurrent responses (TPR); (b) electrochemical impedance spectra (EIS); and (c) Mott-Schottky plots
三、光催化剂的降解性能
与Bi2MoO6和AgI相比,可见光照射下,Bi2MoO6/AgI复合光催化剂表现出更优异的光催化活性(图4a)。孔雀石绿的光催化降解符合伪一级反应动力学方程,BA11催化下的降解速率常数(k)分别是Bi2MoO6和AgI的9.6和10.7倍(图4b)。经过5个连续循环的光催化降解实验,BA11的催化效率几乎没有下降,表明Bi2MoO6/AgI复合光催化剂具有良好的稳定性(图4d)。
Malachite green (MG) was used as a model pollutant to test the activity of the photocatalysts. The presence of photocatalysts significantly enhanced the removal of MG under visible light irradiation. Overall, Bi2MoO6 and AgI showed poor photocatalytic activity with only 29.0% and 49.7% of the MG removed within 40 min. As expected, the Bi2MoO6/AgI nanocomposites exhibited better photocatalytic activity than the individual components (Fig. 4a). The pseudo-first order degradation rate constants (k) of MG in the presence of BA11 was 9.6 and 10.7 times higher than that in the presence of Bi2MoO6 and AgI, (Fig. 4b). The stability and reusability of BA11 were further evaluated by conducting the photocatalytic degradation experiment in five consecutive cycles. As shown on Fig. 4d, BA11 had excellent photocatalytic stability with negligible loss in the removal efficiency of MG over the reuse cycles. Compared to that of the fresh one, the XRD pattern of the used BA11 did not show any significant change after the five cycles of use (Fig. 4e), which confirms that it had good stability in aqueous solution and under visible light irradiation.
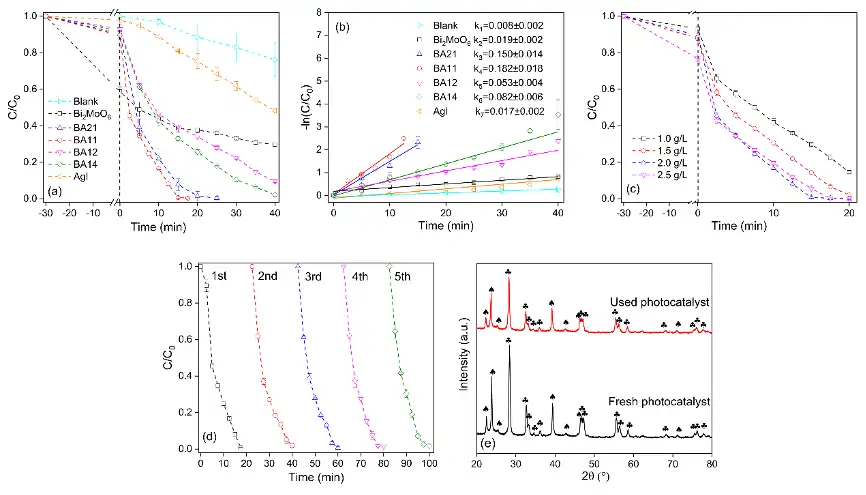
图4 可见光下孔雀石绿的光催化降解
(a)Bi2MoO6、AgI和Bi2MoO6/AgI复合光催化剂的光降解性能;(b)Bi2MoO6、AgI和Bi2MoO6/AgI复合光催化剂对孔雀石绿降解的伪一级动力学拟合常数;(c)催化剂用量对孔雀石绿降解的影响(BA11);(d)BA11的五次循环实验;和(e)五次循环前后BA11的XRD 图谱
Fig. 4 Photocatalytic degradation of MG (20 mg/L) under visible light irradiation.
(a) MG degradation in the presence of Bi2MoO6, AgI, and different Bi2MoO6 /AgI nanocomposites;(b) pseudo-first order degradation kinetics of MG in the presence of Bi2MoO6, AgI, and different Bi2MoO6/AgI nanocomposites; (c) influence of the dosage of BA11 on MG adsorption and degradation; (d) changes in the photocatalytic activity of BA11 over five consecutive use cycles; and (e) comparison of the XRD patterns of the fresh and used BA11.
四、光催化剂降解的机制
电子顺磁共振波谱分析及自由基淬灭实验表明,光照产生的•O2-和•OH是光催化体系中的主要自由基(图5a,b和c)。BA11的光催化机理如图5d所示。在可见光照射下,Bi2MoO6导带上的光生电子与AgI价带上的光生空穴结合,随后,积累在AgI导带上的电子将溶解氧还原为•O2-,积累在Bi2MoO6价带上的空穴将OH-氧化为•OH。在这个过程中,Bi2MoO6导带上的电子与AgI价带上的空穴结合使得Bi2MoO6/AgI具有Z型结构,从而显示出较强的光催化氧化-还原能力。
Electron paramagnetic resonance analysis and free radical quenching experiments showed that •O2- and •OH generated under light irradiation were the main free radicals in the photocatalytic system (Fig. 5a, b, and c). Figure 5d depicts the proposed photocatalytic mechanism of BA11. Under visible light irradiation, the photogenerated electrons on the conduction band of Bi2MoO6 first combine with the photogenerated holes on the valence band of AgI, and subsequently, the electrons accumulated on the conduction band of AgI reduce the dissolved O2 to •O2-, and the holes accumulated in the valence band of Bi2MoO6 oxidize OH- to •OH. During this process, the combination of photogenerated electrons on the CB of Bi2MoO6 with photogenerated holes on the VB of AgI helps the formation of a Z-scheme structure. As a result, Bi2MoO6/AgI nanocomposite possesses high photocatalytic activity with short charge transfer distance.

图5. 光催化机理分析
(a)BA11的捕获实验;(b)DMPO-•O2-的电子自旋共振 (ESR)光谱;(c)DMPO-•OH 电子自旋共振 (ESR)光谱;和(d)光催化机制
Fig. 5 Proposed photocatalytic mechanism.
(a) influence of scavengers on degradation of MG (20 mg/L) under visible light irradiation in the presence of BA11(2.0 g/L); (b) spin-trapping electron spin resonance (ESR) spectra of DMPO-•O2- formed in methanol dispersion (5 mL) in the presence of BA11(1mg) under dark and visible light irradiation; (c) spin-trapping ESR spectra of DMPO-•OH formed in aqueous dispersion (5mL) in the presence of BA11 (1 mg) under dark and visible light irradiation; and (d) proposed mechanism for the generation of reactive species by the Bi2MoO6/AgI nanocomposite, BA11, under visible light irradiation, and the degradation of MG attacked by them.
总结 | Conclusions
Bi2MoO6/AgI光催化剂中的光生载流子按照Z型异质结路径进行传输,使其具有良好的光生电子空穴分离效率和强氧化-还原能力。在可见光照射下,AgI导带上的电子将溶解氧还原为•O2-,Bi2MoO6价带上的空穴将OH-氧化为•OH。因此,Bi2MoO6/AgI光催化剂具有较高的光催化活性。在处理孔雀石绿的过程中也表现出良好的稳定性和可重用性。因此,Bi2MoO6/AgI光催化剂在降解水中有机污染物方面具有实际应用前景。
Benefiting from the Z-scheme structure, Bi2MoO6/AgI heterojunction possesses efficient electron transfer and strong redox activity. Under visible-light irradiation, the electrons accumulated on the CB of AgI would enable the generation of •O2- from dissolved O2, while the holes accumulated on the VB of Bi2MoO6 are capable of oxidizing OH- to •OH. This renders Z-scheme Bi2MoO6/AgI heterojunction with high photocatalytic activity. Meanwhile, Bi2MoO6/AgI heterojunction exhibits good stability and reusability in photocatalytic treatment of malachite green. Overall, the photocatalysts designed and prepared in this work have practical application prospects in the degradation of organic pollutants in water.

扫二维码 | 查看原文
https://www.sciencedirect.com/science/article/pii/S0048969721022981
本文内容来自ELSEVIER旗舰期刊Sci Total Environ第784卷发表的论文:
Liu, J., Wang, G.W., Li, B., Ma, X., Hu, Y.A., Cheng, H.F., 2021. A high-efficiency mediator-free Z-scheme Bi2MoO6/AgI heterojunction with enhanced photocatalytic performance. Sci Total Environ 784, 147227.
https://doi.org/10.1016/j.scitotenv.2021.147227